Hannah Corderman is trying to fill in the blanks in her world, but the blanks are growing bigger. She cannot always hear conversations, so she nods or smiles at what seem like appropriate moments, taking her cues from people around her. Picking out individual words can be hard even though doctors recently turned up the volume on her hearing aids. “There’s a lot missing from conversations—bits and pieces,” she says. “But I make it work.”
Making it work is becoming more difficult. Corderman has an inherited condition called Usher syndrome that is slowly but inexorably robbing her of two major senses. A genetic mutation is starving cells in both her inner ear and her retina of proteins needed to detect sound and light. On top of her hearing loss, her declining vision, which forced her to give up driving at night as a teenager, has recently worsened. Now, at the age of 24, she has blind spots that make it harder to see during the day, too. No current treatment can halt or slow the disease, so Corderman lives with the knowledge that in 10 years, maybe 20 if the deterioration is slow, she could be deaf and blind.
As a child, Corderman’s trouble seemed to be a straightforward hearing deficit. Then one summer in high school, as she looked up at the sky from her home in Needham, Mass., the stars seemed to blink out one by one. She told herself they were probably just covered by clouds. But the problems continued, and eventually doctors diagnosed her with Usher type 2A, a deaf-blind disorder that sets in gradually over many years. She took in the news quietly, she told me when I spoke with her. After absorbing it, she said, she decided to get on with her life. Today, seven years after her diagnosis, Corderman has finished college and works on the marketing side of her family’s construction company, pushing back as much as she can against a disease that she says will not define her.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
There is not much doctors can do to help. Corderman could one day get a cochlear implant, a device that bypasses the hair cells to directly stimulate the auditory nerve and give her some sense of sound. But the device is a relatively blunt fix. For eyes, retinal implants—electrical stimulators for light-detecting cells—are rarely used because they do not come close to actual vision.
Corderman is not a rigorous reader of science journals, but she is aware of several hundred young mice in a few laboratories in Boston, not far from her house. The mice were bred to have auditory disorders like her own—but these mice are getting better. Using an approach called gene therapy, biologists have been dosing them with healthy DNA to make up for bad stretches that produce faulty proteins. In 2017 biologist Gwenaëlle Géléoc of Boston Children’s Hospital and her colleagues reported an “unprecedented recovery” in such mice after the scientists delivered a DNA infusion to the inner ear, restoring the animals’ hearing to near-normal levels. Around the same time, a separate team at Harvard Medical School reported more modest hearing restoration when they used a similar genetic approach in mice with a different hereditary disorder. A third Boston-area group recently employed gene-editing methods to knock out a faulty gene in “Beethoven mice”—rodents with another form of progressive hearing decline named after the deaf composer. The advances are providing hope that for the first time, genetic hearing disorders—some of the most common birth defects in the U.S.—could be treated and even stopped at their source.
Going viral
Gene therapy has had a difficult and checkered past. In an infamous 1999 incident, an 18-year-old liver disease patient named Jesse Gelsinger died in an early gene therapy trial conducted by researchers at the University of Pennsylvania. A virus was used to ferry genes into his body, but the dose and type caused Gelsinger’s immune system to whip itself into a fatal frenzy, attacking his own organs. The tragedy chilled public enthusiasm and funding and made many scientists cautious about going further.

Born with Usher syndrome, Hannah Corderman has a genetic disease that is gradually stealing her hearing and her sight. Credit: Ethan Hill
Others, however, continued to quietly work with this technique, at first focusing on cells and animals, in hopes of developing therapies for complex disorders such as osteoarthritis, cancer and type 1 diabetes. As a safeguard, they lowered the doses of viruses used as delivery vehicles to keep the immune system from overreacting. They also moved away from the virus that had been used with Gelsinger, a member of a group called adenoviruses, and instead experimented with other types. One promising alternative seemed to perform particularly well in these tests: adeno-associated virus (AAV), a gene courier that appears benign to the human immune system because it does not harm our cells. Sometimes the gene-carrying viruses have been prepackaged within cells when injected, a technique that keeps them in a small target area. The field has become about “matching the right vehicle to the right disease and target and understanding dosing and where the viruses are going in the body,” says researcher Cynthia E. Dunbar of the National Institutes of Health, who recently served as president of the American Society of Gene & Cell Therapy.
The improvements have worked. The U.S. Food and Drug Administration has now issued the first wave of approvals of gene therapy for people, indicating that it may be an idea whose time has finally come. In August 2017 the FDA gave a green light to Kymriah, a virus-delivered treatment for a form of childhood leukemia. And in December of that year, the agency approved the first gene therapy for a rare inherited form of blindness. Drug companies and venture capitalists are now pouring a lot of resources into gene therapy, Dunbar says. At the society’s 2018 annual meeting, about 3,400 people showed up. Only 1,200 were in attendance five years earlier.
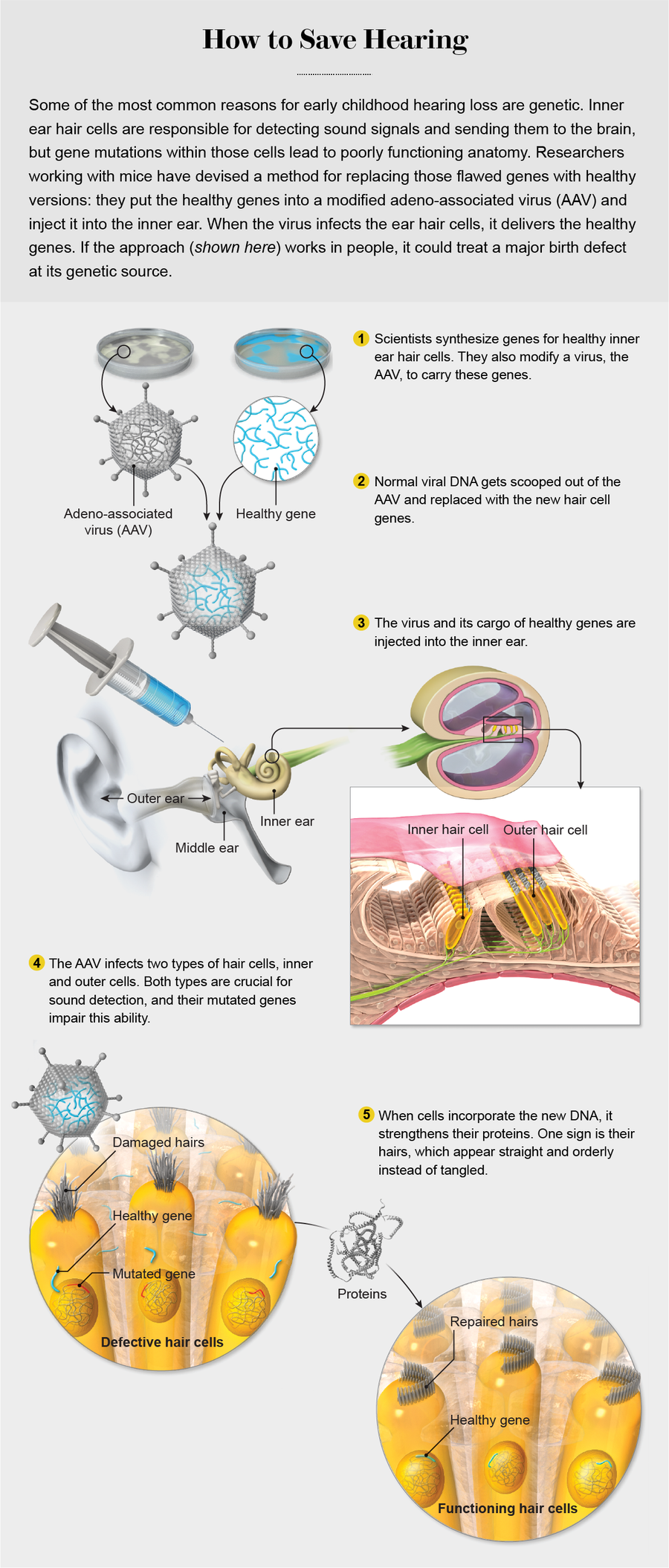
Credit: Emily Cooper
This enthusiasm now extends to hearing loss, a disorder that is often genetic in nature. Though popularly associated with aging or accidents, hearing loss is one of the most common birth defects afflicting people; it affects as many as three out of every 1,000 babies. Genetic flaws are responsible for more than half those cases, including diseases such as Usher syndrome. Usher is a particularly appealing target because each patient has mutations in a single gene, and fixing them could fix the symptoms. Certain forms of the disease, such as Corderman’s, progress gradually, which provides a window after diagnosis when treatment could stop the genetically instigated damage. That damage occurs in hair cells within the inner ear—the hairs pick up sound waves from outside and transmit them to the brain. Corderman and others like her have defective genes that grow weakened hair cells. Those cells are “like a spark plug in a car” says David Corey, one of the Harvard scientists who restored sound sensation to some mice. “Without them, hearing just doesn’t work.”
Gene therapy for these cells is a way to treat the root cause of the disease rather than putting on a high-tech bandage such as a hearing aid. The recent “successes are very impressive and important early promising steps,” says Theodore Friedmann, a pediatrician who helps to lead a consortium of gene therapy researchers at the University of California, San Diego, and was not involved in the work. Mice are not people, of course, and the technique has yet to be tried on humans. But Friedmann says such interventions do, for the first time ever, open the door to treating deafness at the genetic level.
Hair treatment
One morning in 2017 in a lab at Harvard, I watched Bence György lean through that door. He was actually bending over a rodent bred to have faulty hair cell genes. The mouse had been anesthetized, and György, a postdoctoral fellow who works with Corey, made a tiny incision behind the animal’s ear. He pushed past paper-thin pieces of tissue as he searched for a spot in the middle ear called the round window membrane, the portal to the inner ear. When György found the membrane, he inserted a fine needle and started to slowly inject a pale pink solution containing about 200 billion particles of an AAV, each carrying a corrected form of the gene responsible for the mouse’s hearing loss.
Using a virus such as an AAV to ferry that precious cargo boosts the gene’s chances of arriving at its destination in the inner ear because even harmless viruses are good at infecting cells. Researchers have learned that they cannot use just any AAV, however. The hallmark of a successful AAV is the ability to infect not just one but two types of hair cells. Various AAVs are reasonably good at delivering DNA into the innermost rows of hair cells, which communicate with nerve cells, but those same viruses do a poor job of getting into the outer rows of such cells, and those are the ones that amplify sound in the first place. For full restoration of sensitivity, the vehicle needs to get into both types, says Corey, who did some of the crucial research on hair cell function.

USING A VIRUS to ferry healthy genes into deaf mice, Gwenaëlle Géléoc and Jeffrey Holt, researchers at Boston Children’s Hospital, restored a sense of hearing to the rodents. Credit: Ethan Hill
Through a mix of trial and error and some viral gene redesign, scientists hit on a few AAVs that are drawn strongly to the two inner ear targets. They altered proteins that make up the external shell of the virus, molecules that help it bind to a cellular target. Eventually this work yielded a set of shell proteins that appear to match up with molecules on both types of hair cells, allowing the virus to make its way inside. In the paper by Géléoc and her colleagues published in 2017, researchers reported that one of these specially modified AAVs arrived intact in rodents genetically destined to be deaf at birth and produced rows of strong, well-functioning hair cells. Other research groups reported they were able to get related AAVs into the inner ear hair cells of older rodents with ears that more closely resemble those of young children.
Getting in, however, was only part of the hearing therapy problem. The other part was identifying the mutations that led to defective hair cells. Researchers started to do this in the 1990s by identifying families with hearing and vision loss typical of Usher and comparing their genomes. These individuals had several genes that seemed to be involved with both ear and eye development, making them prime suspects. Scientists then bred mice both with and without those suspect mutations to test whether any of the genetic changes were truly responsible for symptoms. The comparison pointed a finger at a few specific alterations. Changes to a gene called USH2A, for example, are behind gradual disorders such as Corderman’s; the nonmutated version of the gene produces healthy hair cells. Each case of the most severe and rapid-onset form of the disease, called Usher type 1, is associated with mutations in one of five different genes, such as one called USH1C.
In the past several years Géléoc’s team put all these pieces together. In the lab, she and her husband, otolaryngologist Jeffrey Holt, and others took an AAV version with a customized shell, scooped out a bunch of its genes related to the life cycle of the virus and replaced them with intact, healthy versions of USH1C. They also added a DNA sequence called a promoter that turns USH1C on in the hair cells. The new gene acted like a booster for the cells when it was delivered. The cells still had the old, faulty DNA that made weak hair proteins, but the addition of well-functioning DNA helped them create a large batch of other healthy proteins that kept the hairs strong.
The team at Boston Children’s Hospital then took the entire package and, using a surgical insertion method similar to the one I watched György use, put it inside Usher mice. Within two weeks the virus had infected at least some of the ear hair cells; by six weeks it had penetrated around 80 percent of them. More to the point, these animals reacted to sounds. One of the main tests of restored ear function involves exposing the rodents to a sudden, startling noise to see if they jump. Many did: the once deaf mice could hear.
Sound was not the only important check. Hair cells do another key job in the body: they create a sense of balance and orientation as the hairs flex within the fluid of the inner ear, sending signals about their position to the brain. Mice with hairs damaged by Usher often have difficulties with movement and figuring out where they are in a space. Instead of sniffing around a cage, these rodents cower in the corner. And although mice are natural-born swimmers—ready to thrive the first time they are placed in the water—an Usher mouse will frantically paddle in circles for a few seconds, struggling to determine which side is “up.” (Scientists quickly rescue it before the animal becomes too distressed.) If the gene therapy mice at Boston Children’s Hospital truly had restored hair cell function, these problems should have gone away.
When I visited Holt and Géléoc’s lab right after my trip to Harvard, I saw mice that acted remarkably like, well, mice. Rodents that had gene therapy two months prior nosed around their environment; their behavior in open spaces and in the water was virtually indistinguishable from their normal counterparts. They moved so easily that I repeatedly asked Holt and his team if they were sure these were the mice with mutations. The scientists, who have a careful system for tracking their animals, assured me each time that I was looking at damaged and treated rodents.
The size of the problem
Despite this success, there are still several vexing difficulties to sort out before these viruses are tried on people. One problem is that the current AAVs are too small. Although they are big enough to carry genes to correct Usher type 1C—the disorder that responded so well in mice trials—many other types involve much larger genes. Corderman’s form of Usher, for instance, involves a gene that is simply too big to squeeze into the available AAV storage space. “It would be like trying to fit a size-14 body into size-4 pants,” Dunbar says.
One work-around would be to slice up the large gene into several chunks that could be carried through the ear in multiple viral vehicles. Each of the gene bits would have sticky ends so that when they arrived at their destination, they could stick themselves back together like Velcro. For example, Corderman’s type of Usher gene is so large that it would need to be cut into three parts. For the approach to work, all three virus carriers must get into the inner ear hair cells, and then the three stretches of DNA need to find one another within a cell and paste themselves back together. The highly specific nature of DNA sequences makes this possible—normally in the body, stretches of the genetic alphabet interact only with complementary stretches—but nonetheless difficult to pull off, Géléoc says.
Other options include using a bigger non-AAV virus, tweaked to avoid widespread immune system alarms, or avoiding viruses altogether and trying to deliver the genetic goods within nanoparticles (minuscule lab-made objects that can penetrate cells). Various researchers, including Holt and Géléoc, are also exploring a way to remove the problem gene and replace it with the right one using the gene-editing technique CRISPR-Cas9. Usher is a recessive disorder, caused by two copies of the faulty gene. If researchers could cut out one of these genes and swap in a healthy dominant one, that new gene could take over and swamp the negative effects of the remaining recessive DNA.
No one has yet achieved this feat using CRISPR, however; the method seems better suited to cutting things out than to sticking them in. For that reason, current CRISPR work in mice looks like it would be more effective for rare hearing issues caused by one faulty gene instead of two. The problem gene could simply be knocked out, allowing the remaining gene to do its job properly. The experiments on Beethoven mice used that approach. Although it worked well, other scientists using CRISPR have seen that it caused unwanted DNA changes on other, nontarget cells. For that reason, nobody thinks gene editing of this type is ready for people. Viral vehicles still seem to be the front-runners for therapy.
No matter what delivery system or other solution researchers devise, it will not help many people unless hearing loss diagnoses improve among infants, who would benefit most from early interventions. In the U.S., most newborns are screened for hearing loss, yet they are rarely diagnosed with a specific disorder or its underlying genetic cause. That is exactly what happened to Corderman, who was not diagnosed with a genetic problem until high school. That will need to change so that children can get treated properly.
Gene therapists do think they will be able to treat children—and perhaps sooner rather than later. “It’s exciting that there are actual products wending their way through the system,” Dunbar says. Elizabeth Olmsted-Davis, a gene therapy researcher at the Baylor College of Medicine, says she is not surprised that gene therapy for other ailments has recently become a clinical reality and that new treatments will eventually follow the same path. “The therapies on the horizon are the culmination of decades of work by talented researchers who saw the potential that these approaches hold,” she says.
Although interest in gene therapy is clearly surging in the research community, Hannah Corderman is not waiting for the wave to reach her. With or without medical advances, she is determined to live a full life. She has booked multiple trips to watch the Northern Lights in case, one day, she can no longer see them. “I’d say my outlook on life has completely changed because it’s like I only have so much time to get things done,” she says. She has become an advocate for fellow patients, too. Corderman says she has realized that speaking out about the genetics problems can help push research further and “potentially inspire others to live their lives and not let this disease hold them back.” Losing her hearing, she says, does not mean retreating into silence.

